The struggle to preserve human health is one of the major challenges that we face throughout history. Medical advances significantly improve the overall standard of living, directly affecting life expectancy, except for relieving disease right here, right now.
Today, medicine is on the verge of amazing breakthroughs. Modern doctors can benefit from developments in brain-computer neural interfaces, xenotransplantation of organs (transplantation of organs from animals to humans), and the development of telesurgery. However, every year, healthcare professionals face new unresolved problems. Paradoxically, the emergence of some of these problems, such as the development of antibiotic-resistant bacteria, is partly facilitated by medicine itself.
We have compiled the top-5 most significant and promising medical technologies and approaches.
Breakthroughs in Bioinformatics in Virology
Bioinformatics is booming in the 21st century. This area of computational microbiology focuses on analyzing biological data structures by encoding them and processing the resulting data sets. With the help of math algorithms and computing technologies, bioinformaticians build mathematical models and observe the most implicit relationships between the smallest cellular structures of RNA and DNA (ribonucleic acid and deoxyribonucleic acid) which form the genome of all biological organisms.
Identifying the relationships in the genome and the processes responsible for the behavioral mechanisms of protein structures expands the understanding of the pathogenic bacteria and viruses nature. One of the recent achievements of bioinformatics methods in virology was the successful deciphering of the nature of the mechanism responsible for the development of HIV Tat proteins, published in the Journal of Virology.
Many protein structures (domains) possess several sets of functions at the same time. The Tat protein of HIV is also one of them. Its protein domain consisting of nine amino acids is responsible for three functions. For example, when attached to RNA, the virus is able to create full-fledged structures of new viruses, infecting more and more healthy cells. Another function of HIV Tat-proteins is the Nuclear Localization Signal (NLS), emitted by the viral protein when it reaches the cellular nucleus and triggers the mechanism of virus multiplication. The mechanism of the third function of Tat-proteins has not yet been studied, but it is responsible for forming the Nucleolar Localization Signal (NLS).
source: rcsb.org
The construction of math models of the virus protein domain has made it possible to identify a set of mechanisms for its communication with the nucleus of the infected cell. The prospect of understanding the basic functioning of viral proteins opens the door to the foreseeable possibility of blocking their mechanism of operation by preventing HIV division, especially in the early stages of infection.
Uncontrolled mutations of RNA viruses can sometimes harm themselves. For example, HIV, which has a high mutagenesis rate, accumulates a threshold of mutations over time and produces deformed proteins unable to create new infected cells.
This effect was first discussed by German biochemist Manfred Eigen back in 1971. His scientific work about virus mutagenesis allowed for many studies of the “error catastrophe” effect in viruses – a particular condition in which a virus mutates so much that it ceases to be dangerous. American researchers discovered that HIV starts to produce “broken” proteins only once 18 mutations accumulate in its genome.
There is every reason to believe that the cumulative effect of the “bug disaster” may eventually create viruses such as SARS CoV-2, HIV, and several other RNA viruses less dangerous (up to the point of being completely harmless). However, until this happens evolutionarily, the availability of vaccines will be necessary.
mRNA Vaccines and the Foreseeable Victory over Malaria
A good example of the matrix-RNA coding technologies development is the vaccines against the SARS virus CoV-19 and its strains from Pfizer and Moderna. These vaccines are innovative because they do not contain an attenuated version of the virus (like vector vaccines) but rather a synthesized sequence of its RNA code. In other words, the vaccine shows the body how to trigger a mechanism to produce a small number of viral proteins. They, in turn, are attacked by the human immune system, training and gaining the necessary level of immunity against the causative virus.
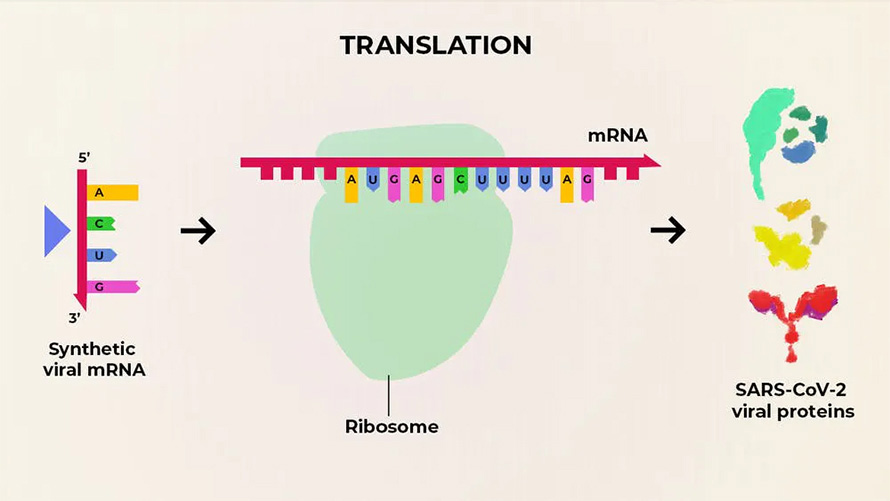
source: The Conversation, CC BY-SA
Matrix RNA vaccines are good because the structure of the synthesized RNA matrices can be easily altered. It allows for making the most relevant serums when new virus strains emerge. Matrix vaccines are planned to be used not only for creating immunity against COVID-19. Similar vaccines are being studied to prevent some types of cancer, HIV, and several bacterial infections like malaria.
Another success in vaccination was the invention of the malaria vaccine RTS,S/AS01 (pharmaceutical name Mosquirix), developed by GlaxoSmithKline. The inventors of the vaccine possess more than 30 years of experience in developing immune protection against the malaria-causing bacterium P. falciparum. In October 2021, the World Health Organization (WHO) recommended using the vaccine for African children, who suffer particularly from malaria: it kills 260,000 children under the age of five in Africa alone annually.
The antibody production mechanism in response to the malaria-causing Circumsporozoite Protein (CSP) is not fully studied, but the vaccine can already guarantee a 30-50% immune response in vaccinated children. The GAVI Vaccine Alliance, the Global Fund to Fight AIDS, Tuberculosis and Malaria, and UNITAID are funding further research on RTSS. Therefore, hopefully, humanity will see more effective analogs in the near future.
Advances in Transplantology and the Development of Telemedicine
Xenotransplantology has been another area where genome intervention has brought tremendous success. On January 10, 2022, the website of the University of Maryland Medical School published the news about the first successful human transplant of a genetically modified animal heart. David Bennett, a 57-year-old patient, who could have faced imminent death from acute arrhythmia, had received a pig heart transplant. Unfortunately, on the evening of March 8, David Bennett passed away; the doctors did not detect an exact cause of the patient’s death.
To minimize the risks of the organ rejection by the new owner, the doctors had to perform some manipulations with ten genes: four pig genes were artificially switched off, while six human genes, on the contrary, were activated. The intervention in the pig genes was performed to reduce the risks of rejection of the heart by the immune system of its new owner, as well as stop the process of growth of the pig organ in the human body.
source: medschool.umaryland.edu
The development of xenotransplantation can have both positive and negative consequences. It’s a fact that a pig liver transplanted to humans is invulnerable to the two most dangerous hepatitis viruses, B and C. The bad news is that it is unknown how infections, which are harmless in animals, will behave in the human body. There is also no clarity about the functionality of animal organs; placed in the human body, they may not fully perform all life-supporting functions. Studies on primates have confirmed this. Until the whole issue is fully observed, xenotransplant surgeries will remain the exception rather than a widespread solution.
Another promising area of modern surgery is telesurgery, which has become possible thanks to the active introduction of 5G wireless high-speed connectivity technologies. It involves remote intervention by the surgeon via robotic surgeons from the patient’s side and manipulator controls from the doctor’s side. In doing so, the low signal latency of the latest generation communication networks allows the surgeon to be at a significant distance from the person being operated on. In March 2019, Dr. Lin Zhipei of the PLA General Hospital in Beijing successfully performed remote brain surgery on a patient 1,800 miles away from the surgeon.
In common sense, telemedicine involves consultative and diagnostic activities in addition to surgical interventions. In the era of the COVID-19 pandemic, most national health systems followed this path, providing consultations to their patients without leaving the confines of their homes. An important factor in the success of this strategy will be the high level of collection and processing of patient biomaterials (medical tests), which, together with the patient’s complaints, will help make the right diagnosis.
Neural Interfaces Integrated with the Brain
The process of improving the integration of artificial neural brain-computer interfaces (BICs) is not over yet. Presumably, such interfaces will be able to help paralyzed people regain mobility by controlling robotic prostheses or to interact with a computer using thought and effort of will.
This is possible by integrating a chip capable of reading the electrical signals coming from the brain’s neurons into the brain of a paralyzed person. A prolonged practice process allows the person to begin interacting with the chip’s neural network, communicating through brain impulses with the software algorithm, and giving it commands as to what action the prosthetic-manipulator should perform.
One of the successful testers is Nathan Copeland, a volunteer with 90% body paralysis. Nathan has agreed to participate in a research program on a neurofeedback interface consisting of a group of implant sensors implanted in the motor and tactile cortex of his brain. The presence of feedback in the chip allows Copeland not only to direct the manipulator with the power of thought but feel the touch of the prosthesis to objects, their density, and even their structure, making him the world’s first “tactile cyborg.”

source: media-cldnry.s-nbcnews.com
It is worth noting that neural interfaces can do more than affect the quality of life of paralyzed people. They can also use innovative techniques in the treatment of a number of mental illnesses. A neurochip implanted into the brain of Sara (the real name of the patient was not disclosed) suffering from terminal clinical depression helped perform deep brain stimulation (Deep Brain Stimulation, DBS) of the brain’s areas responsible for the onset of depressive states.
depression was read by a special neurochip connected to the brain. Using AI algorithms was able to decipher Sarah’s brain impulses, understand the causes of her particular type of depression, and help the brain develop a neural response to the signals of depression. These breakthroughs were made possible through the efforts of Helen Mayberg, a neurosurgeon and pioneer in deep brain stimulation.
Bacteria, Antibacteria, and Charles Darwin
Antibiotic-resistant bacteria were the third cause of death worldwide in 2019, according to the 2022 Lancet Global Antimicrobial Resistance Report. The following six pathogens were found to be the most resistant to most current antibiotics: Staphylococcus aureus and pneumococcus, E. coli and Pseudomonas aeruginosa, Akinethobacterium baumannii, and Klebsiella pneumoniae.
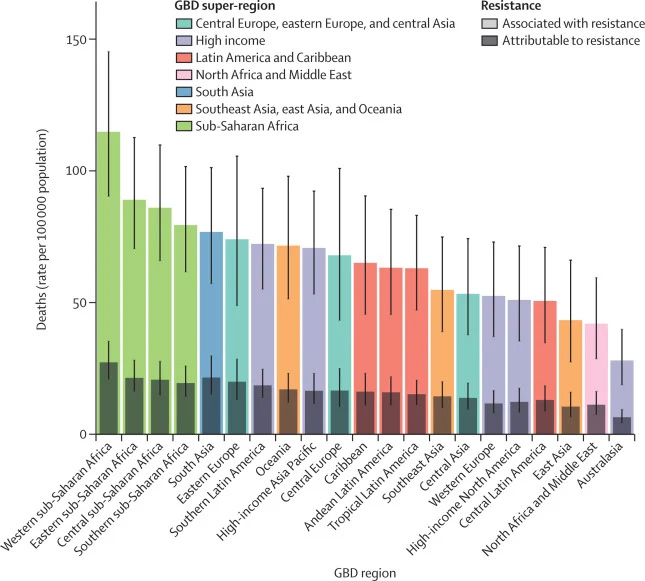
source: Antimicrobial Resistance Collaborators, The Lancet, 2022
So, why do bacteria become immune to antibiotics? Natural selection, the evolution of bacteria, plays a major role in this process. In places with high concentrations of both bacteria and antibiotics, there is always a percentage of microbes capable of adapting in an aggressive environment. Such bacteria survive, as well as retain their ability to divide. In 2016, biologists at Harvard Medical School were able to recreate this mechanism experimentally. In just eleven days, they artificially bred colonies of bacteria that were 1,000 times more resistant to the antibiotic than their predecessors. To do this, they placed the bacteria in an artificial container divided into nine sectors.

source: wired.com
11 days of bacterial survival at Harvard Medical School:
- Sectors 1 and 9 (start of experiment) – natural medium;
- Sectors 2 and 8 – x10 antibiotic concentration in the medium;
- Sectors 3 and 7 – x100 antibiotic concentration in the medium;
- Sectors 4, 5, 6 (end of the experiment) – x1000 antibiotic concentration in the medium.
Interestingly, the most antibiotic-tolerant bacterial colonies were fully uncompetitive among their congeners. The most antibiotic-resistant bacteria were simultaneously the weakest in the evolutionary race outside the environment containing the antibiotic.
Over-the-counter and chaotic usage of antibiotics is a major social factor influencing the development of super bacterial colonies. This is why the decision to take antibiotics should always be made based on medical advice. In addition, localizing infections in hospitals and preventing them from escaping through increased sanitary control requirements plays an important role in curbing the development of bacterial resistance. Developing the level of vaccination against bacterial infections, improving the quality of drinking water, and abandoning antibiotics in livestock production can significantly slow down the emergence of new drug-resistant microbial mutations.
The situation with resistant bacteria might be solved not only by new types of more active antibiotics but also by the possibility of artificially interfering with the matrix RNA and DNA of resistant bacteria to edit their genome further. Medical-scientific research in this area will be able to reverse the evolution of pernicious pathogenic structures.






